Bucky Fuller
© Thomas Wilson Shawcross 23 October 2005

First Day of Issue of the U.S. commemorative postage stamp honoring Richard Buckminster Fuller was 12 July 2004.
What species of tree makes the best firewood? Apple? Ash? Cedar? Hickory?
If I know you, this is precisely what you were wondering about just now. Well, as it turns out, about twenty-five years ago, when I lived in Michigan, The New Yorker magazine published an article that discussed this!
Since I had two fireplaces in my Michigan home and a recently-purchased cord of firewood stacked in my garage, I figured I should read this story, so that I would know what kind of wood I should order next time. Not knowing any better, I had bought generic “wood,” but thanks to this magazine story, I would make an informed choice next time! I learned that some people preferred hard woods, such as ash, oak and hickory, while others preferred more aromatic woods, such as apple, cedar, and mesquite, but even though different species of trees varied in hardness and in fragrance, the chemistry involved in the burning of firewood was the same. The process of burning logs involves the release of its moisture content as water vapor (up to 60% of the weight of a “green” log can come from water – which is why one should try to burn only “dry” split logs that are about 20% water). The non-water part of logs consists mostly of hydrocarbon compounds, which leads us to consider carbon itself.
Carbon is a unique element because of its ability to form covalent bonds that are strong and stable. As we all remember, covalent bonds involve the sharing of electrons. Carbon has only four electrons in its outermost energy level, but there is room for eight. Think what this can mean!
Put another way, carbon is the Tara Reid of atoms – the true Party Girl of the Periodic Table of Elements.
As such, it is not unusual to find a carbon atom simultaneously “holding hands” with four hunky hydrogen atoms (we call this resultant compound “methane,” or CH4). The fact that carbon bonds so easily with common elements such as hydrogen, oxygen, nitrogen, and phosphorous is interesting in itself and has much to do with the fact that we humans (and all other living things) are carbon-based life forms. But wait, there’s more! Carbon can also form chains of almost unlimited length by bonding to other carbon atoms (personally, I wouldn’t mind seeing that). In fact, it can bond in straight chains of single, double, or even triple covalent bonds or combinations thereof. As The New Yorker story explained, when a log burns, its hydrocarbon compounds react in a similar way to how gasoline reacts when exposed to a flame. In fact, gasoline is chains of hydrocarbons. As such, a log can be thought of as containing a solid form of gasoline.
That statement caught my imagination. I had never thought of logs as gasoline. When I had looked at a forest, I had seen only the trees. Now, I saw gasoline! As the more complex hydrocarbons in the tree heat up when exposed to flame, the chains break up and ignite, starting with the one-carbon methane gas, and including many other combinations, such as two-carbon ethane, three-carbon propane, four-carbon butane, etc. (and don’t forget eight-carbon octane)!
OK, so that was an interesting story, but I had other things to do, so I didn’t dwell on it. Frankly, I gave little more thought to it until a year or so later, when another article in The New Yorker grabbed my attention.
This other article contained a Bucky Fuller story (by now, I suppose you were wondering why I called this firewood-carbon story “Bucky Fuller”). Bucky Fuller had been a guest of an editor of The New Yorker. After dinner, they had retired to the den, where there was a fireplace. The young son of the editor came by to say goodnight, and when he looked at the burning logs in the fireplace, he asked what fire was.
Oh, how I wished I had been there! This would have been my big chance to impress Bucky Fuller, the possessor of one of the greatest minds of our time!
I had been so impressed by Bucky Fuller when he spoke at the campus of Southern Illinois University in Edwardsville (I saw Janis Joplin perform there too, but that is another story). It would have been a thrill to turn the tables and impress Buckminster Fuller!
I still recalled that firewood-gasoline story, and I ached for the chance to tell it to him. But wait! It turns out that the editor also remembered that story (possibly, he had edited it), and the editor related how he was about to reveal that logs were another form of gasoline . . . rats!
But then it got really interesting.
Before the editor (or me, in my fantasy world) could impress Bucky Fuller, Bucky asked the boy if he remembered when the logs in the fireplace had been part of a tree that had lived in the backyard. The boy remembered.
“Remember how the sun had shone on the leaves of the living tree, and it had grown and made these logs?” Bucky asked. “Well, now the sun is coming back out of the logs.”
Oooh! Good one. This explains why Bucky Fuller invented the geodesic dome and I didn’t. He knew how to see things in a different way.
Many years later (ok, it was in 1995), I found myself working as a business consultant to a company in Alberta, Canada. My client had exclusive rights to build the pipelines that transported natural gas from northern Alberta, and in return got to “skim the cream” from the natural gas, which is mostly methane. The more complex ethane gas was the “cream” as it has more of the highly coveted Tara Reid, er, carbon atoms and is therefore a much better monomer for building the more complex polymers of polyethelene and polystyrene.
The polyethelene pellets made by my client were then sold to manufacturers of shrink wrap food packaging, squeezable bottles, and grocery bags. The polystyrene pellets were sold for the manufacturing of polystyrene foam (known as Styrofoam when made by DuPont), and rigid products such as compact disc cases, and computer cabinets.
In an interesting twist of fate, Buckminster Fuller and carbon were to become forever associated through the research of Richard E. Smally, Robert F. Curl, Jr., and Harold Kroto, who won the 1996 Nobel Prize in Chemistry for their discovery of fullerenes, a family of highly symmetrical carbon-cage molecules whose prototypical member is C60, known as Buckminsterfullerene, or a "Buckyball."
A Buckyball is the roundest and most symmetrical large molecule known to man. It has astounding properties of shock resistance, superconductivity, and, of course, has its carbon atoms arranged in the shape of a hollow geodesic dome.
Wish I’d thought of that . . .
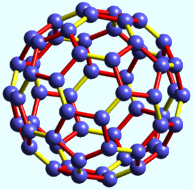
Buckyball with single- (red) and
double- (yellow) bonds highlighted

First Day of Issue of the U.S. commemorative postage stamp honoring Richard Buckminster Fuller was 12 July 2004.
What species of tree makes the best firewood? Apple? Ash? Cedar? Hickory?
If I know you, this is precisely what you were wondering about just now. Well, as it turns out, about twenty-five years ago, when I lived in Michigan, The New Yorker magazine published an article that discussed this!
Since I had two fireplaces in my Michigan home and a recently-purchased cord of firewood stacked in my garage, I figured I should read this story, so that I would know what kind of wood I should order next time. Not knowing any better, I had bought generic “wood,” but thanks to this magazine story, I would make an informed choice next time! I learned that some people preferred hard woods, such as ash, oak and hickory, while others preferred more aromatic woods, such as apple, cedar, and mesquite, but even though different species of trees varied in hardness and in fragrance, the chemistry involved in the burning of firewood was the same. The process of burning logs involves the release of its moisture content as water vapor (up to 60% of the weight of a “green” log can come from water – which is why one should try to burn only “dry” split logs that are about 20% water). The non-water part of logs consists mostly of hydrocarbon compounds, which leads us to consider carbon itself.
Carbon is a unique element because of its ability to form covalent bonds that are strong and stable. As we all remember, covalent bonds involve the sharing of electrons. Carbon has only four electrons in its outermost energy level, but there is room for eight. Think what this can mean!
Put another way, carbon is the Tara Reid of atoms – the true Party Girl of the Periodic Table of Elements.
As such, it is not unusual to find a carbon atom simultaneously “holding hands” with four hunky hydrogen atoms (we call this resultant compound “methane,” or CH4). The fact that carbon bonds so easily with common elements such as hydrogen, oxygen, nitrogen, and phosphorous is interesting in itself and has much to do with the fact that we humans (and all other living things) are carbon-based life forms. But wait, there’s more! Carbon can also form chains of almost unlimited length by bonding to other carbon atoms (personally, I wouldn’t mind seeing that). In fact, it can bond in straight chains of single, double, or even triple covalent bonds or combinations thereof. As The New Yorker story explained, when a log burns, its hydrocarbon compounds react in a similar way to how gasoline reacts when exposed to a flame. In fact, gasoline is chains of hydrocarbons. As such, a log can be thought of as containing a solid form of gasoline.
That statement caught my imagination. I had never thought of logs as gasoline. When I had looked at a forest, I had seen only the trees. Now, I saw gasoline! As the more complex hydrocarbons in the tree heat up when exposed to flame, the chains break up and ignite, starting with the one-carbon methane gas, and including many other combinations, such as two-carbon ethane, three-carbon propane, four-carbon butane, etc. (and don’t forget eight-carbon octane)!
OK, so that was an interesting story, but I had other things to do, so I didn’t dwell on it. Frankly, I gave little more thought to it until a year or so later, when another article in The New Yorker grabbed my attention.
This other article contained a Bucky Fuller story (by now, I suppose you were wondering why I called this firewood-carbon story “Bucky Fuller”). Bucky Fuller had been a guest of an editor of The New Yorker. After dinner, they had retired to the den, where there was a fireplace. The young son of the editor came by to say goodnight, and when he looked at the burning logs in the fireplace, he asked what fire was.
Oh, how I wished I had been there! This would have been my big chance to impress Bucky Fuller, the possessor of one of the greatest minds of our time!
I had been so impressed by Bucky Fuller when he spoke at the campus of Southern Illinois University in Edwardsville (I saw Janis Joplin perform there too, but that is another story). It would have been a thrill to turn the tables and impress Buckminster Fuller!
I still recalled that firewood-gasoline story, and I ached for the chance to tell it to him. But wait! It turns out that the editor also remembered that story (possibly, he had edited it), and the editor related how he was about to reveal that logs were another form of gasoline . . . rats!
But then it got really interesting.
Before the editor (or me, in my fantasy world) could impress Bucky Fuller, Bucky asked the boy if he remembered when the logs in the fireplace had been part of a tree that had lived in the backyard. The boy remembered.
“Remember how the sun had shone on the leaves of the living tree, and it had grown and made these logs?” Bucky asked. “Well, now the sun is coming back out of the logs.”
Oooh! Good one. This explains why Bucky Fuller invented the geodesic dome and I didn’t. He knew how to see things in a different way.
Many years later (ok, it was in 1995), I found myself working as a business consultant to a company in Alberta, Canada. My client had exclusive rights to build the pipelines that transported natural gas from northern Alberta, and in return got to “skim the cream” from the natural gas, which is mostly methane. The more complex ethane gas was the “cream” as it has more of the highly coveted Tara Reid, er, carbon atoms and is therefore a much better monomer for building the more complex polymers of polyethelene and polystyrene.
The polyethelene pellets made by my client were then sold to manufacturers of shrink wrap food packaging, squeezable bottles, and grocery bags. The polystyrene pellets were sold for the manufacturing of polystyrene foam (known as Styrofoam when made by DuPont), and rigid products such as compact disc cases, and computer cabinets.
In an interesting twist of fate, Buckminster Fuller and carbon were to become forever associated through the research of Richard E. Smally, Robert F. Curl, Jr., and Harold Kroto, who won the 1996 Nobel Prize in Chemistry for their discovery of fullerenes, a family of highly symmetrical carbon-cage molecules whose prototypical member is C60, known as Buckminsterfullerene, or a "Buckyball."
A Buckyball is the roundest and most symmetrical large molecule known to man. It has astounding properties of shock resistance, superconductivity, and, of course, has its carbon atoms arranged in the shape of a hollow geodesic dome.
Wish I’d thought of that . . .

Buckyball with single- (red) and
double- (yellow) bonds highlighted


1 Comments:
Hi Tom, I, too, heard Buckminster Fuller speak at the Religous Center at SIU-Edwardsville. An interesting man! Nick and I lived only a 1/2 mile from the River Festival site, and hear many concerts through our bedroom window. We lived about a mile from the religous center.
Mary Byron
Post a Comment
<< Home